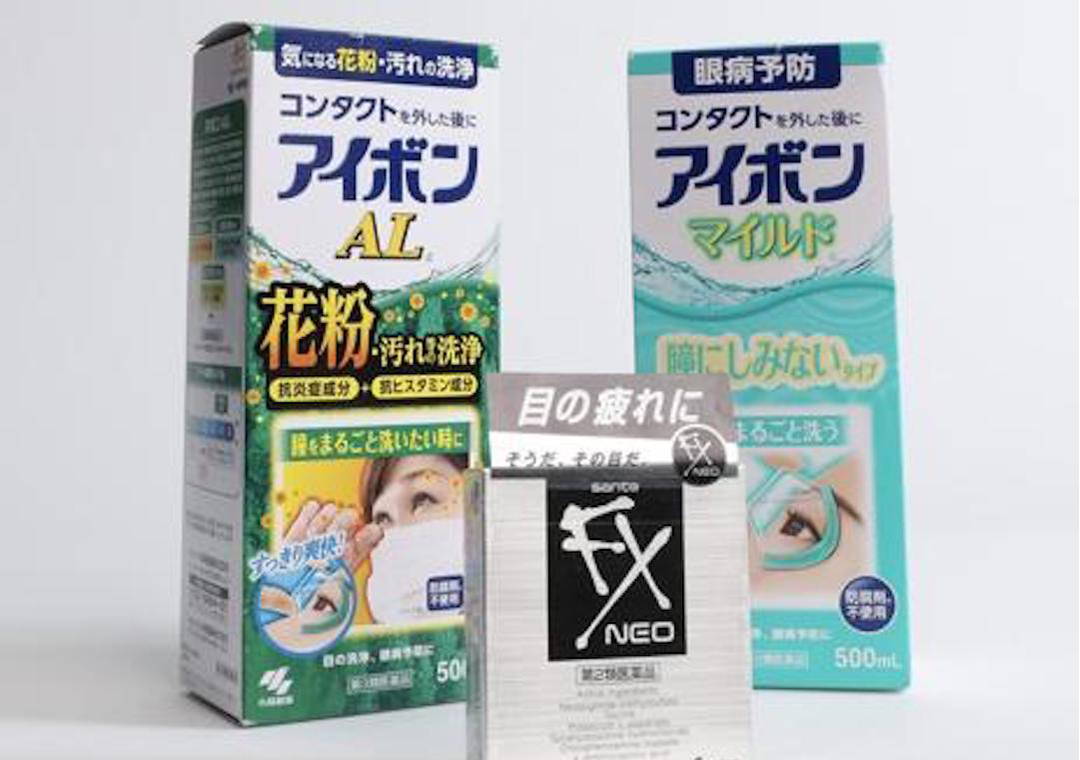
The eye drop for sale at the popular health and beauty store chain risked causing iris cysts, retinal detachment and “glaucoma attack,” Health Canada inspectors determined after visiting a Mississauga location earlier this year.
They also found several types of an eyewash brand that contain a prescription-only drug that can be absorbed through the tear ducts into the bloodstream and cause nausea, muscle weakness and a skin rash.
None of the products have been approved for sale by Health Canada but they were available at Kiokii And…, a chain that is rapidly expanding throughout the GTA. The inspectors seized the illegal products and issued a public health advisory — the seventh since 2019 for these products.
Just three weeks later, the products were still for sale. Reporters were able to purchase them upon request from Kiokii staff.
As regulators struggle to get a handle on the massive flow of health and beauty products streaming onto store shelves, a Star/Investigative Journalism Bureau (IJB) investigation found more than 100 products that Health Canada officials confirmed have not been approved for sale or assessed for safety.
We found them not only at Kiokii, but also for sale in Walmart and Amazon online stores, as well as at Loblaw-owned grocery chain T&T Supermarket stores across the GTA and Alberta.
More than 50 of those products — including children’s cough medication, skin creams and sunscreens — contain ingredients that require a Health Canada licence or identification number for sale that appeared nowhere on the packaging or on Health Canada’s public databases of products approved for sale.
A handful, including eye drops, headache medication, hemorrhoid ointment and kids canker sore patches, were for sale despite having ingredients that require a prescription from a doctor and oversight from a pharmacist at the point of sale. Such regulatory breaches expose consumers to health risks.
“There are not many categories of issues higher from a risk perspective … than finding a prescription drug in a non-prescription product,” a Health Canada spokesperson said.
“The sale of unauthorized health products is illegal in Canada … Health Canada thanks the Toronto Star and Investigative Journalism Bureau again for bringing these products to its attention.”
Retailers vow to remove products
While not all of the products identified by reporters may be dangerous to consumers, they reveal a federal regulatory system overwhelmed by highly popular health and beauty products that have not met federal regulations.
All four retailers promised to address the regulatory issues, and they pulled dozens of products from shelves and websites.
“We’re still learning about regulations,” said Kiokii director Jing Peng, who noted that the company had recently acquired three of its seven stores from another chain. “We are trying to make sure everything is right. We’re expanding very fast … I will really fix it.”
In a written statement, a senior Kiokii manager said the chain is “in the process of addressing the matters with Health Canada and committed to full compliance with the regulations.”
Representatives for T&T Supermarket said the products flagged by reporters would be removed from its shelves. They included sunscreens as well as herbal and over-the-counter medications that lacked proper labelling and didn’t have licence approval from Health Canada.
“As is industry standard, we rely on the importer of record to ensure their products meet Canadian compliance guidelines and will be in touch with our vendors,” the store said in a written statement. “Many of these products are very popular and beloved by customers around the world … Our customers ask for them by name and we are hopeful manufacturers can continue to work with regulators on updating labelling and getting recognition for their highly sought-after products.”
On July 27, Health Canada issued a public warning about three “unauthorized Japanese-labelled” drugs inspectors seized from a North York T&T Supermarket that could pose “serious health risks.”
The products — a hemorrhoidal ointment, anti-itch cream and canker sore medication for children — list prescription drugs on their labels.
They are among the products reporters asked Health Canada about during reporting of this story.
Friday’s Health Canada public advisory says, “The company advised Health Canada that these products were only being sold at this location.”
Products sold online at Walmart and Amazon
Reporters provided Walmart Canada with a list of four products — three sunscreens and one acne treatment — reviewed by Health Canada. All four were missing required licensing for these products.
The company said the products were sold by third-party vendors on its website. “After a review, we are in the process of removing these products from our site and notifying the sellers.”
Amazon Canada said in a statement that it requires its selling partners to “comply with applicable Canadian laws,” and the products listed by reporters “have been removed from sale.” The products included five sunscreens and two dietary supplements that also require a licence to be sold in Canada.
Health Canada acknowledged the challenges of detecting and removing illegal health and beauty products from store shelves.
“While our inspectors are continuously conducting site visits across the country, our ability to follow up on certain reports and complaints is limited when we do not receive all the necessary information,” it reads. “Health Canada will continue to respond to complaints and to act when it finds unauthorized health products on the market.”
Some products were flagged by experts and Health Canada as particularly concerning because they were marketed towards kids or contained ingredients that posed a risk to consumers.
‘It’s a Wild West’
EVE Quick DX, a Japanese pain medication that reporters found at Kiokii And…, contains a prescription-only ingredient called allylisopropylacetylurea, a sedative with hypnotic effects. The medication does not appear in Health Canada’s drug and health product database, which details all products and drugs approved for sale.
Dr. David Juurlink, an internist and division director of clinical pharmacology and toxicology at the University of Toronto who reviewed the product list for this investigation, said some “have the potential to cause harm” even if used as directed by the package. Juurlink says the risk is also amplified “by the fact that the products can be purchased without interacting with a pharmacist.”
Even at recommended doses, EVE Quick DX “can cause severe and potentially life-threatening allergic reactions,” Juurlink said.
EVE Quick DX was purchased for $25.99 at Kiokii’s Yonge Street location. When asked what the ingredients of the pain medication are, an employee in the store said they weren’t sure but to take two pills after eating.
“If these (products) are being sold outside of a place where you can reach a pharmacist to give you advice, then it’s possible it’s a Wild West because you don’t know what these different ingredients may do or not do for you,” said Dr. Joel Lexchin, a Toronto physician and pharmaceutical policy expert.
Lexchin reviewed the labels of products found at retailers by reporters for this investigation.
“I’ve been a doctor for 45 years and some of these ingredients, I had no idea what they were or what the alleged uses were,” he said.
Pabron Kids Cold Granules, a children’s cold medication found at three T&T Supermarket locations in the GTA, lists no Health Canada identification numbers, dosage information or instructions for safe use on its package. It also appears nowhere in Health Canada’s drug product database.
The brightly coloured packaging with labelling mostly in Japanese also features a smiling cartoon cat holding a strawberry and the word “kids” in English.
It contains chlorpheniramine, an allergy drug that has “no basis in cough and cold medication,” according to Lexchin, who reviewed the product’s ingredient list.
‘Dangerous’ to have products with no guidance on use
Reporters also found dozens of products for sale that appear to breach federal labelling rules by not having dosage, ingredient and usage details in English and French.
Heather Boon, a professor at the University of Toronto’s Leslie Dan Faculty of Pharmacy who studies natural health products, calls it “dangerous” to have products available for consumer use without the appropriate guidance.
“We have a regulatory system in Canada that everybody has to adhere to and it’s concerning that these products appear to be circumventing that.”
Health Canada confirmed it has an “active case” against Kiokii following its May advisory, but said it “cannot provide feedback regarding the status of ongoing non-compliance verification activities.” There is no public record of any penalty imposed on the chain.
The sale of unregulated products also undermines those who do follow the regulatory framework.
“It’s concerning to see non-compliant products in the market, not only because of the potential risks to consumer safety, but also because they undermine market fairness,” said Steve Li, a regulatory affairs consultant.
“It’s important to adhere to regulations for the benefit of Canadians and to ensure a level playing field in the industry.”
This article is also available on the Toronto Star website.
- Feds vow to probe findings of IJB/Star/TVO investigation on mental health care for Indigenous people - 4 November 2024
- ‘Deeply troubling’: Ontario chiefs call for reforms to federal health program after Star/IJB investigation - 3 November 2024
- ‘I am deeply troubled’: Head of Ontario law society speaks out after Star/IJB investigation into sexual harassment among lawyers - 25 October 2024